Navigation
Mechanics
Heat and Thermodynamics
Heat and TemperatureTemperature ScaleMeasurement of heat energy and Specific heat energyLatent HeatSaturated and Unsaturated VapourRelative humidity and dew pointThermodynamics.Reversible isothermal and adiabatic changesFirst Law Of ThermodynamicsHeat Transfer ConductionConvection RadiationSolar Constant and Important NotesGas lawsKinetic theory of gasesSecond Law of thermodynamicsCarnot's engineExpansion of SolidExpansion of Liquid and Gas
Magnetism
Geometrical Optics
Wave Optics
Electrostatics
Current Electricity
Second Law of thermodynamics
Second Law of thermodynamic:
1) Kelvin Planck statement:
- It is impossible for engine to convert all the heat energy into work without rejecting some energy to sink i.e. no engine will have 100% efficiency.
- Presence of sink is essential for continuous conversion of heat into work.
2) Clausius statement:
- It is impossible to absorb heat energy from cold body and reject to hot body without doing work on it i.e. self acting refrigerator is impossible.
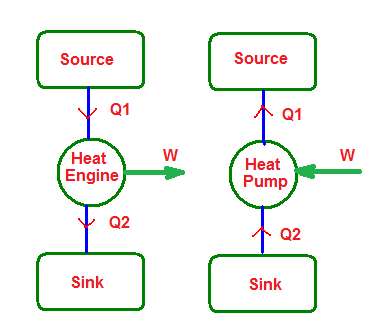
Heat engine:
- Any device which converts heat energy continuously into mechanical work
- Its main parts are:
i)Source: A hot body at a constant high temperature (T1) from which the heat engine can draw heat (Q1).
ii)Sink: A cold body at a constant low temperature (T2) to which any amount of heat can be rejected.
iii)Working Substance: Working substance is an ideal gas which on being supplied with heat performs mechanical work.
Efficiency of heat engine:
- External work obtained to the heat energy absorbed by the working substance from the source.
- Denoted by ɳ
- ɳ=%
- ɳ=%
- ɳ=%
- ɳ=%
- When heat engines are placed in series then sink of 1st engine act as source for 2nd engine and so on.
- Efficiency of heat engine always less than 1 or 100%