Reversible isothermal and adiabatic changes
Thermo dynamical process:
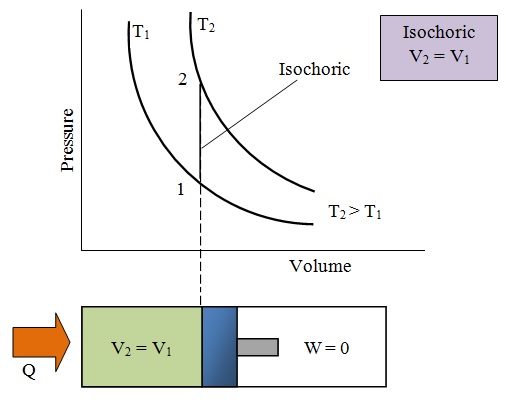
1) Isochoric Process:
- Volume remains constant
- Work done (dw)=0
- Heat supplied = change in internal energy: dQ=dU
- ndT=dU
2) Isobaric Process:
- Pressure remains constant
- dQ=dT+ PdV

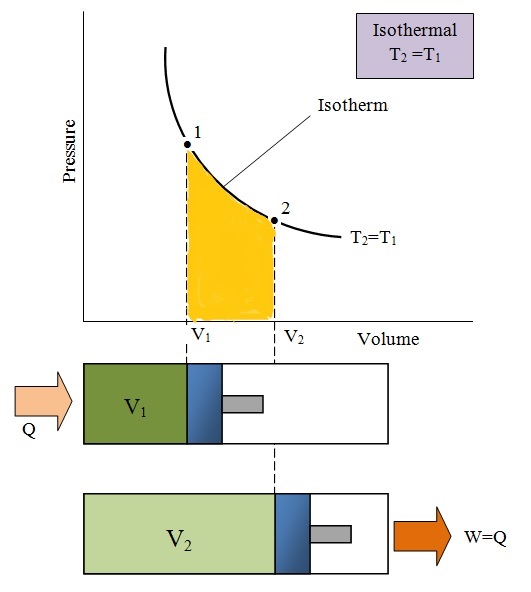
3) Isothermal Process:
- Temperature remains constant. i.e. dT=0
- For this process cylinder with conducting wall is used and ideal gas filled inside is allowed to expand or is compressed very slowly.
- Eg: Melting process and boiling process
- Specific heat capacity during isothermal process is infinity
- Change in internal energy(du)= 0
- Gas Equation:
- Slope of curve
- Work Done (w) =nRT ln
= =
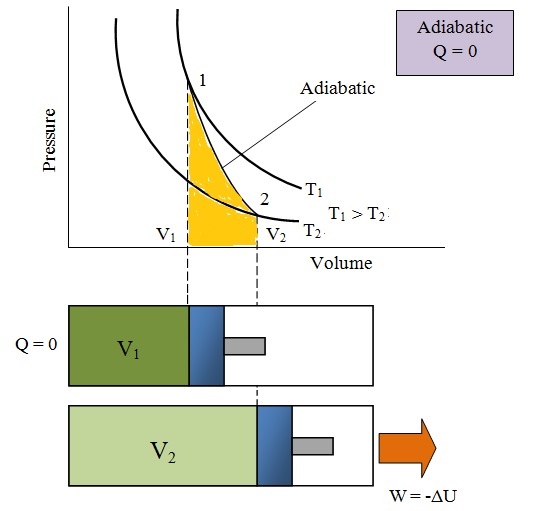
4) Adiabatic Process:
- The process in which exchange of heat energy is zero i.e. dQ=0
- So, dW=-dU i.e. work is done by gas on the expense of internal energy so cooling is observed after adiabatic expansion
- Fast process in which wall of cylinder is perfectly insulator
- Specific Heat capacity of gas is 0.
- Eg: Propagation of sound wave, sudden bursting of tire, the compression stroke in an internal combustion engine.
- Slope of curve
- Gas equation is
1)
2)
3) =
- Work done (w) = =
- For heat capacity: dQ=mdT
C=
- Heat capacity = 0
Cyclic Process:
- Complete a closed cycle
- Change in internal energy is zero
- Work done (w) = Area of closed loop in PV diagram
Reversible Process
- An infinitesimally slow expansion and compression of an ideal gas at a constant pressure
- At mechanical processes take place under the action of conservative force. No loss of energy due to conduction or radiation during the cycle of operation.
- All thermal processes taking place at infinitesimally slow rate
Irreversible Process:
- Cannot retrace in the opposite order by reversing the controlling factors
- Eg: Rusting of iron, dissolve of soap in water, decay of matter, flow of current through a conductor, etc.

Limitation of 1st law of thermodynamics:
1) Does not indicate the direction of heat transfer.
2) Does not indicate as to why heat energy developed in the target cannot be converted back into mechanical energy of the bullet enabling it to fly back.
3) Does not give to what extent the mechanical energy in obtained from the heat energy.
4) 1st law is silent about the efficiency of the heat engine.
Important notes:
1) Work done:
- During expansion: W(isobaric) >W(isothermal) > W(adiabatic)
- During compression: W (adiabatic) > W(isothermal) > W (isobaric)
2) Pressure is constant during state change.
3) Generally if no any information of gas is given we have to use i.e. diatomic.
4) The expansion of gas against zero external pressure is known as free expansion.
5) The gas has the greatest internal energy whereas the solid has the least.
6) Specific heat of saturated vapor pressure is negative.
7) Work done during isothermal expansion is more than work done during adiabatic expansion if the initial and final volumes are same.
8) Adiabatic curve is steeper than that of isothermal curve.
9) In a cyclic process, the internal energy of a gas remains constant.
10)In adiabatic process, the temperature of an isolated system changes
11)When air of atmosphere rises up, it cools. Why? (Expansion occurs in the air, it becomes cool.)
12)Internal energy of an ideal gas is wholly kinetic in nature and function of temperature.
13)Two isothermal curves cannot intersect each other.
14)When the cold air blowing across the mountain tops descends into the valley, it is adiabatically compressed. Consequently, the temperature in the valley is increased.