Measurement of heat energy and Specific heat energy
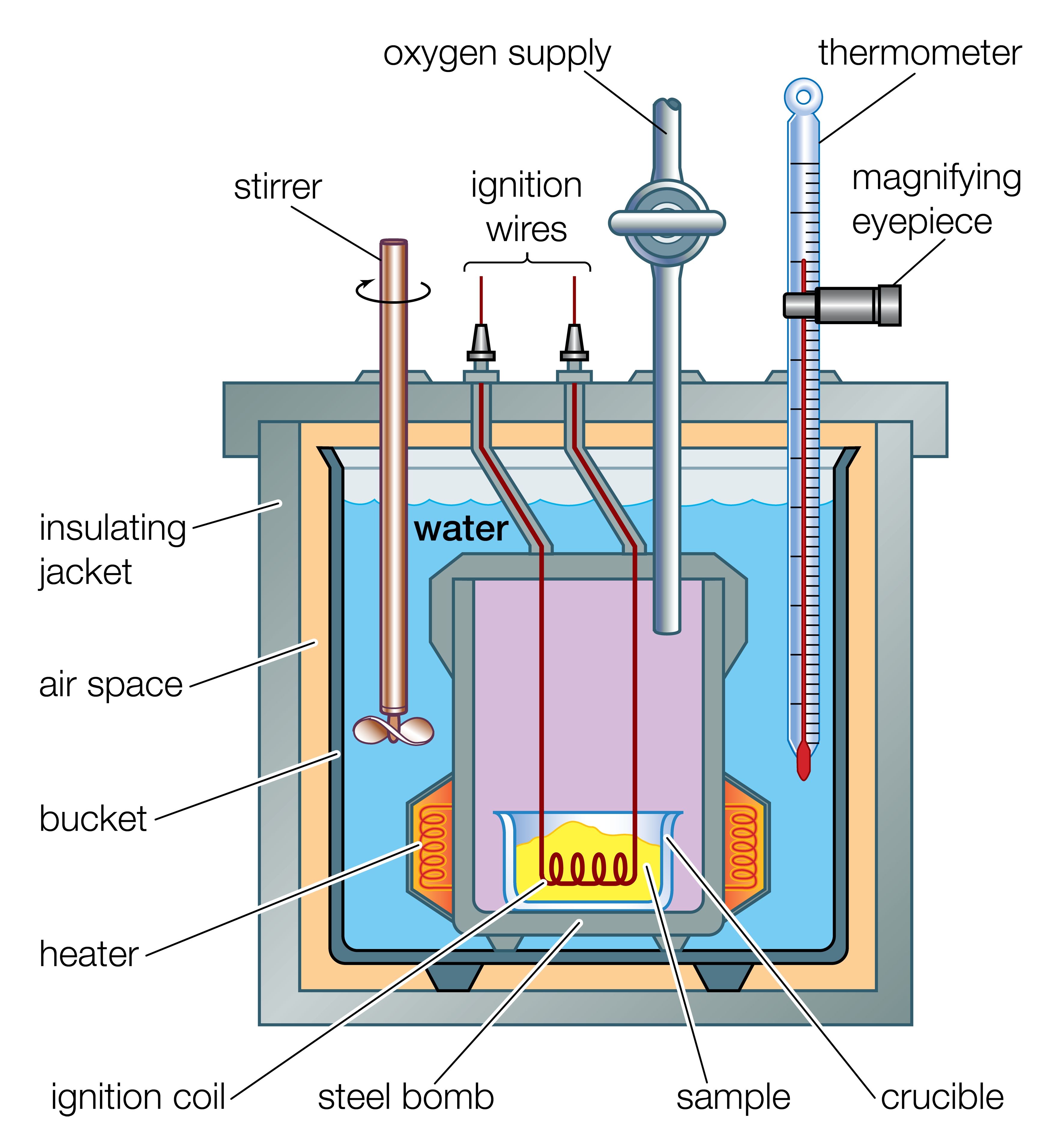
Calorimetry
- It is the experimental technique for quantitative measurement of heat exchange.
- Calorie is the unit of heat in CGS system and joule in SI-unit.
- One calorie is the amount of heat necessary to raise the temperature of one gram of water from 14.5C to 15.5C
- One calorie is equal to 4.2 J
Specific heat capacity
- Amount of heat required to rise the temperature of unit mass of body through 1C
- Its unit is
- where s is specific heat capacity and other symbol has usual meaning.
Heat Capacity or thermal capacity (C)
- Amount of heat required to increase temperature of a body through one degree
- Heat capacity (C)=ms
Water equivalent
- Mass of water that will absorb or lose the same quantity of heat as the substance for the same change in temperature.
- where is water equivalent, is specific heat of water and m and s are mass and sp. heat of other body.
- In CGS in CGS system
Principle of Calorimetry
Heat loss by a body=Heat gain by another body {based on principle of conservation of energy}
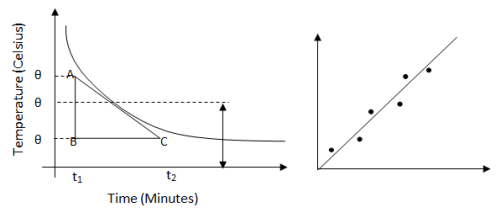
Newton's Law of Cooling
- The rate of loss of heat of a body is directly proportional to the difference in temperature between body and surrounding.
- It is valid for small difference in temperature between body and surrounding
- Where -ve sign indicate loss of heat on increasing time, K = Proportionality constant.
- ) .
- After integrating both sides we get
Note:
We see that the rate of temperature fall is inversely proportional to the linear dimension of the object. This is because mass is proportional to the volume and therefore, the rate of fall of temperature of the body is proportional to the ratio of its surface area and volume or inversely proportional to linear dimensional. So, a small body cools faster than a larger one and therefore, a small burning coal of fire can be picked up faster than a larger one. Similarly, a small baby must be thoroughly wrapped up by clothes than a growth up.
The law is applicable for still air only for excess temperature of 20C to 30C. It is also applicable to excess temperature of 50C to 300C at forced convection of air. At very high temperature, the law does not hold true
Notes:
- Water equivalent and heat capacity of a body are numerically equal.
- Tea gets cooled when sugar is added to it.
- Specific heat of a solid varies with temperature.
- Calorimeter are made up of metal with low specific heat capacity.