Navigation
Mechanics
Heat and Thermodynamics
Heat and TemperatureTemperature ScaleMeasurement of heat energy and Specific heat energyLatent HeatSaturated and Unsaturated VapourRelative humidity and dew pointThermodynamics.Reversible isothermal and adiabatic changesFirst Law Of ThermodynamicsHeat Transfer ConductionConvection RadiationSolar Constant and Important NotesGas lawsKinetic theory of gasesSecond Law of thermodynamicsCarnot's engineExpansion of SolidExpansion of Liquid and Gas
Magnetism
Geometrical Optics
Wave Optics
Electrostatics
Current Electricity
Latent Heat
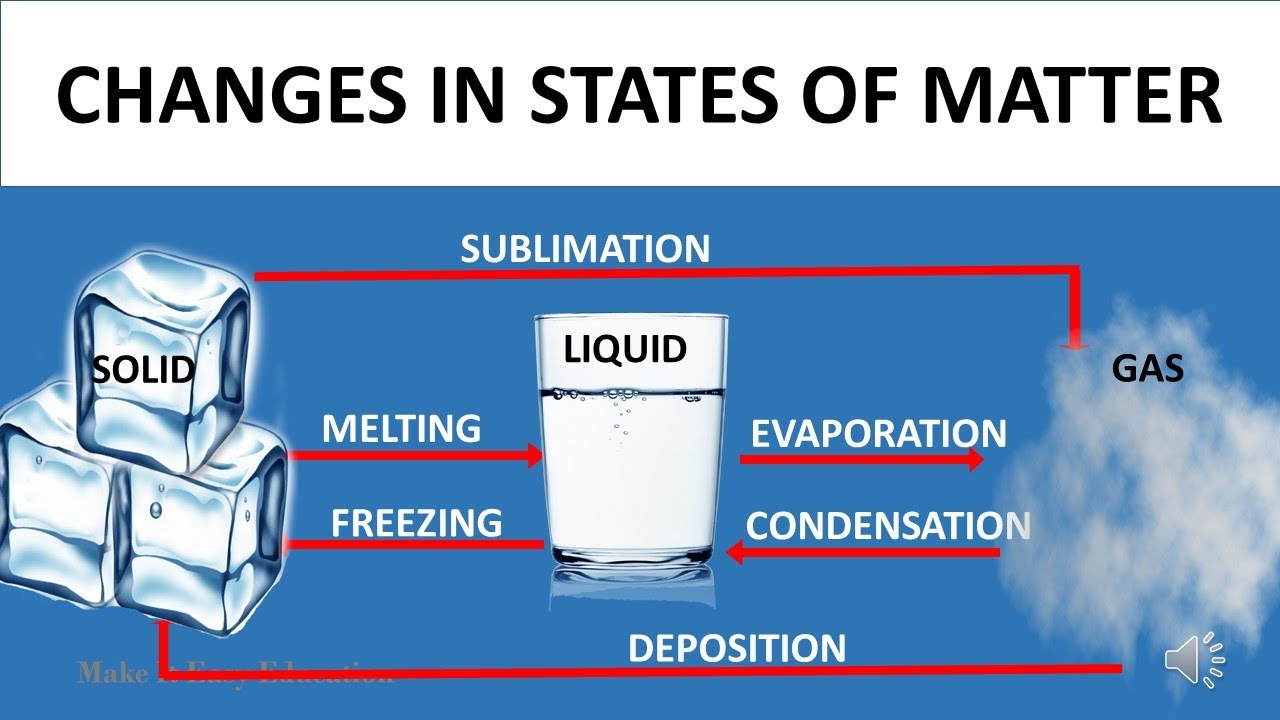
Change of State
- A change in state from solid to liquid on heating is fusion or melting. It occur at certain constant temperature.
- Similarly, a change from liquid to vapor on heating is vaporization. It also occur at certain constant temperature.
Note:
Specific heat capacity during change in state is infinite and 0 for adiabatic process.
Latent heat
- It is the amount of heat required to change the state of unit mass of the substance from solid to liquid or from liquid to vapor without any change in temperature.
Latent Heat of Fusion
- It is the amount of heat required to change the state of unit mass of the substance from solid to liquid at constant temperature.
- The latent heat of fusion of ice is in SI-unit or 80 calin CGS system.
Latent Heat of Vaporization
- It is the amount of heat required to change the state of unit mass of the substance from liquid to gas at constant temperature.
- The latent heat of vaporization of water is in SI-unit or 540 calin CGS system.
Mechanial equivalent
- The heat energy developed equivalent to mechanical energy is called mechanical equivalent.
- W=J.H , where, W is mechanical energy measured in joule, H= Heat energy measured in calorie and J = Joule's mechanical equivalent of heat
Super Cooling:
- When a liquid is cooled gently, it is found experimentally that the liquid may cool even several degrees below its freezing point without solidification. Such a liquid below its freezing point is called super cooled liquid
- Water can be cooled to -20C without forming ice, but even a gentle disturbance can start solidification and immediately the temperature rise to 0C.
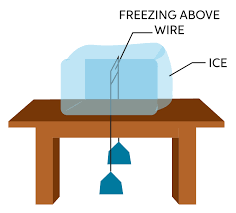
Pressure and melting points
- If solid expands on solidification, the increase of pressure lowers their melting point.
- If solid contract on solidification, the increase of pressure increases their melting point.
Relegation:
The phenomenon of melting of ice due to compression and freezing after removal of applied pressure is called relegation.
Note:
- Latent heat is called hidden heat.
- Evaporation takes place in all temperature and depends on area of liquid surface, temperature and pressure of the liquid, nature of the liquid, effect of wind and humidity of air.
- Boiling is rapid and noisy process which occur at constant temperature.