Navigation
Mechanics
Heat and Thermodynamics
Heat and TemperatureTemperature ScaleMeasurement of heat energy and Specific heat energyLatent HeatSaturated and Unsaturated VapourRelative humidity and dew pointThermodynamics.Reversible isothermal and adiabatic changesFirst Law Of ThermodynamicsHeat Transfer ConductionConvection RadiationSolar Constant and Important NotesGas lawsKinetic theory of gasesSecond Law of thermodynamicsCarnot's engineExpansion of SolidExpansion of Liquid and Gas
Magnetism
Geometrical Optics
Wave Optics
Electrostatics
Current Electricity
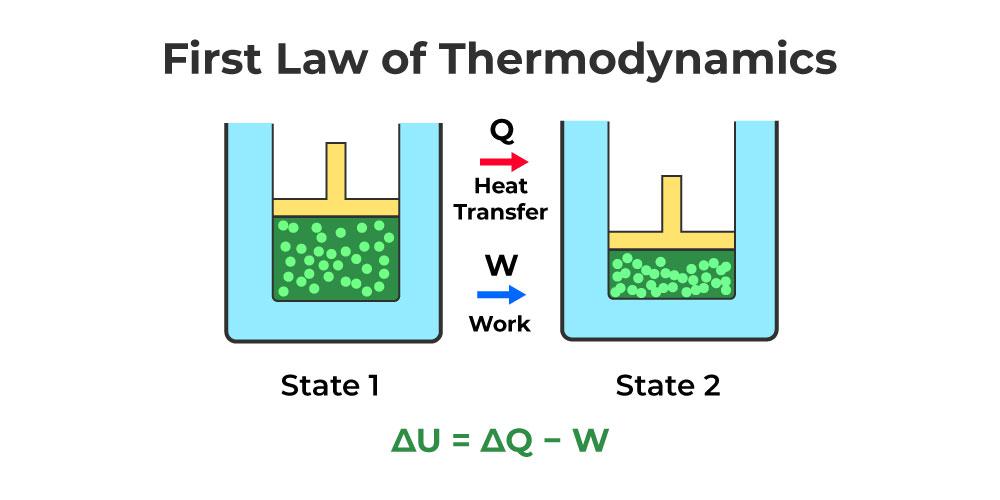
First Law Of Thermodynamics
First law of thermodynamics:
- When heat energy is given to a system then some part of heat energy supplied is used to change the internal energy of system and rest of energy is used to do external work.
- ∆Q=∆u+∆w
Note:
- For cyclic process, the change in internal energy of the system is zero because the system is brought back to the initial condition. Therefore, dU=0 and from the first law of thermodynamics,
- dQ= du + PdV =0+dW= dW
Molar Heat Capacities:
1) Molar heat capacity at constant pressure (Cp)
- Heat required to rise the temperature of one mole of gas through 1 degree C at constant pressure. Its unit is J/(molK)
- Heat required (dQ)=n dT
2) Molar heat capacity at constant volume(Cv):
- Heat required to rise the temperature of mole of gas through 1 degree C at constant volume. Its unit is J/(molK)
- Heat required (dU) = ndT
Mayer`s Formula:
- =R
Specific heat capacities
1) Specific heat capacity at constant pressure ():
- Heat required to rise the temperature of unit mass of gas through 1 degree C temperature at constant pressure.
- Heat required (dQ)=m dT
- =M
2) Specific heat capacity at constant volume ():
- Heat required to rise the temperature of unit mass gas through 1 degree C temperature at constant volume.
- Its unit is J/(kg K)
- Heat required (du)=m dT
- =M Hence,
Note:
- Heat required to rise certain temperature at constant pressure is always greater than heat required to rise same temperature at constant volume. So gas has two types of heat capacities
- i.e.
- Because in constant pressure, internal energy and work done both is done.