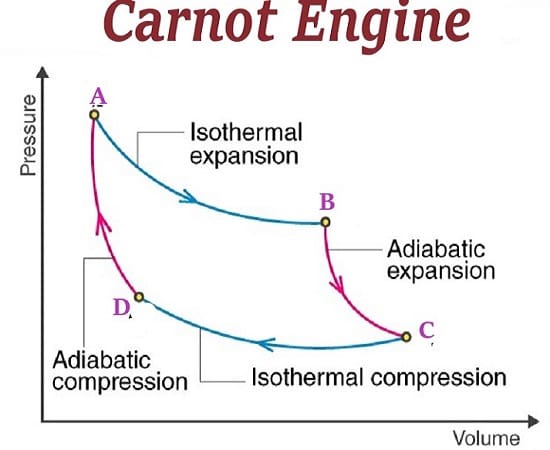
Carnot's engine
Carnot`s engine:
- An ideal cycle of operation for a heat engine.
- Essential parts:
i)Source of heat: Hot body of infinite thermal capacity and maintained at a fixed temperature, from which the working substance draws heat without changing its temperature.
ii)Cylinder: Cylinder is fitted with a perfectly non-conducting and frictionless piston enclosing an ideal gas. Its bottom is a perfect heat conductor whereas the walls are perfect heat insulator.
iii)Sink of heat: Should be in fixed lower temperature and has the infinite thermal capacity.
iv)Working substance: An ideal gas.
- A cycle of four operation consisting of two isothermal processes and two adiabatic process makes a complete Carnot`s cycle.
- Efficiency of Carnot`s engine. (ɳ)=
- In adiabatic compression if
ɳ={}%

Note:
- Carnot engine is perfectly reversible because:
i) No friction between the cylinder and the piston.
ii)The operations on the gas should be performed very slowly.
iii)Lose of heat due to conduction is prevented.
- Efficiency depends on the temperature of the source and that of the sink but does not depends upon the nature of the working substance.
- As is always less than , so efficiency of a heat engine is always less than one or efficiency cannot be 100%.
Refrigerator:
- Operates in a manner opposite to that of a heat engine.
- Coefficient of Performance (): Ratio of the amount of heat absorbed from the cold body to the work done is running the machine.
- heat is absorbed from cold reservoir on doing work ‘’ on it then heat is rejected to hot reservoir.
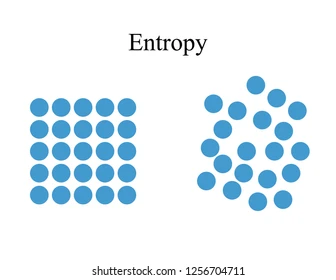
Entropy:
- The measure of disorder of molecule of system is called entropy.
- On increasing the disorder, the entropy of system increases
∆S=
∆Q=T∆S
- The change in entropy during change in state of matter ∆S=
- Where +ve sign indicates heat absorbed and –ve sign indicates heat evolved.
- When temperature of body changes from then
The change in entropy (∆S) =ms ln
Kelvin Temperature Scale:
- The ratio of any two temperatures on this scale is equal to the ratio of heats absorbed and rejected by a Carnot reversible engine working between these two temperatures.
- temperatures are measured in terms of work and hence this scale is also known as work scale of temperature.
Notes:
1) Efficiency of Carnot`s engine is independent of the nature of the working substance.
2) Efficiency of Carnot heat engine is 100% only if the temperature of the sink is zero Kelvin which is not possible.
3) The efficiency of heat engine is more in hilly areas than in the planes.
4) Atmosphere is the sink in a steam engine.
5) As the refrigerator works its coefficient of performance goes on decreasing.
6) It is possible to convert work into the energy completely but the reverse is not possible.
7) Total change is entropy of the whole system in a Carnot`s cycle is zero.
8) Energy while burning petrol or diesel engine is input power.
9) A diesel engine has higher efficiency than that petrol engine.